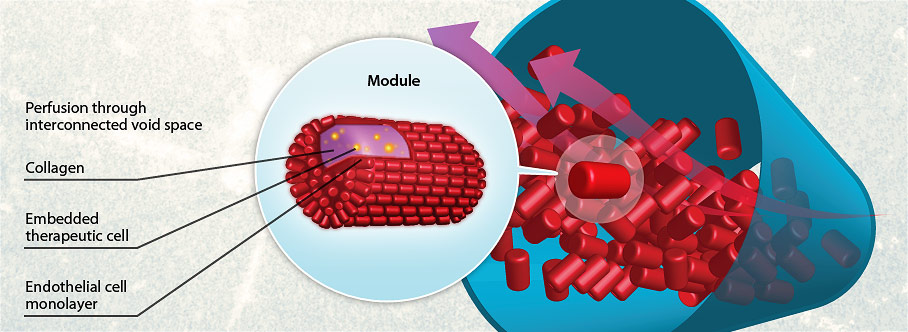
Modular tissue engineering is the practice of designing sub-unit tissue structures, each with a defined function, which assemble together to create a larger therapeutic tissue (i.e. a “bottom-up” approach). Modular tissues are based on the porous structure that results from the random packing of a column or tube with solid objects (here, short cylindrical rods). On a very much larger scale, such packed columns are standard pieces of chemical engineering process equipment. Because of the narrow channels in such columns, mass transfer coefficients are relatively high, making them efficient separating devices – or here in our research, ideal structures to deliver cells sensitive to adequate nutrient exchange. In past work, functional cells (eg cardiomyocytes, liver, fat, or insulin producing cells) have been encapsulated in collagen gel rods (~200-400 µm diameter with variable aspect ratio). These collagen rods were further seeded with endothelial cells (to minimize thrombogenicity and promote vascular integration and tissue organization) or embedded with mesenchymal stromal cells (to enhance the associated regenerative microenvironment). The interstitial gaps among the rods form interconnected channels which became lined by the endothelial cells. In early iterations of the technology, the resulting endothelial cell lining enables whole blood to percolate around the rods and through these interstitial channels.
Artificial modular tissue constructs for treatment of type 1 diabetes
More recently, such modular tissues have been adapted for the use of subcutaneous pancreatic islet transplantation, since it was realized that modules are also injectable and can be remodeled so as to integrate with the host vasculature creating a chimera of host and donor vessels. In the principal manifestation, Islet aggregates can be kept whole or dispersed throughout the collagen rods which are then seeded with endothelial cells. The assembled vascular structure promotes islet health, function, and integration – while the distinct modular nature of the collagen rods allowed manipulation of “islet” cells in novel “pseudo-islet” tissue constructs, paving the way for future studies to optimize a “designer” islet for transplantation.
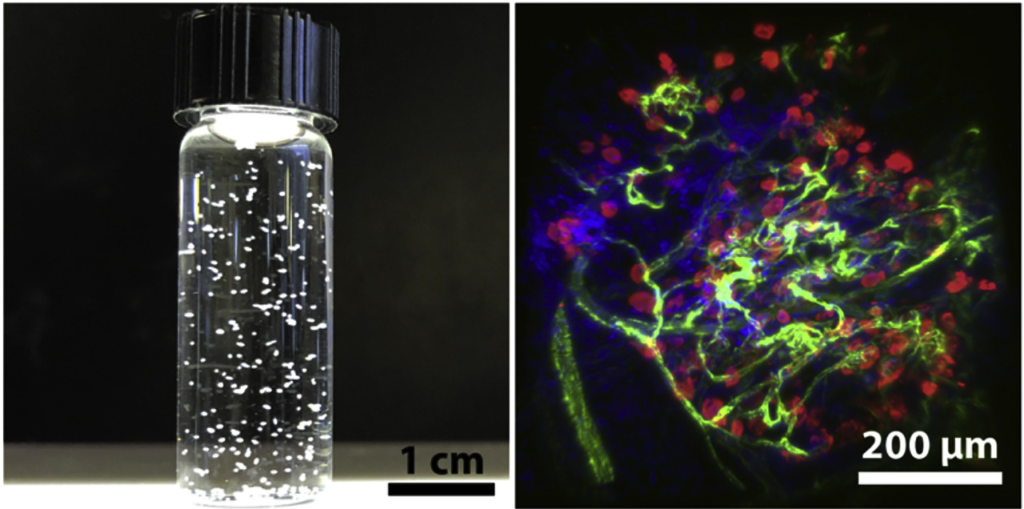
“Pseudo-islets”
Artificial micro-tissues constructed from dissociated islet cells using modular tissue engineering. Image adapted from Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy.

